Share your perspective and help improve the health of our community! We are conducting a Community Health Needs Assessment (CHNA) to help us better understand the health needs of the community our health system serves. Learn more and take the short survey.
HIV Screening Tests and False Positives
Every year over 30,000 Americans receive a new diagnosis of HIV. This has been remarkably stable since the beginning of the epidemic despite very effective treatment, screening of our blood supply and specific populations at risk. Since 2019 the US Preventive Services Task Force and the CDC have recommended screening of all patients between ages 13 and 65 at least once for HIV. They have also recommended screening before and after these ages and repeated testing if the patient is at higher risk for acquiring HIV.
People at higher risk include men who have sex with men, patients who use IV recreational drugs, patients who exchange sex for drugs or money, patients with sexually transmitted diseases (STI) or who have a partner with an STI, patients requesting STI testing, patients with an HIV positive partner and patients with multiple sex partners.
Most HIV is transmitted early in the disease when viral loads are high and the patient is unaware of their status. Finding HIV positive patients and getting them on treatment before they put another person in this position is the best way that we can break this cycle.
I do not believe any outside clinic network does as good a job of implementing useful guidelines as the Valley clinic network. Given this, I believe our providers are helping us reach the goal of reduced HIV incidence by testing a lot of people. Testing a lot of people, however, has a downside. We have definitely seen an increase in inquiries regarding false positive HIV screening tests. Generally, this is what it looks like:

What does this mean? Why is it happening?
Above is the screening test that Labcorp uses. It is the Advia centaur HIV Ag/Ab Combo (CHIV) Assay, which was created for ease of use in screening large numbers of individual patient samples with good sensitivity. It looks for only one common antibody and antigen for both HIV 1 and 2. Because of this, it has a false positive rate of about 2/1000 due to cross reacting antibodies not related to HIV. Given the large number of unselected screening tests we are now doing, it is not surprising that we are seeing periodic false positives with this assay.
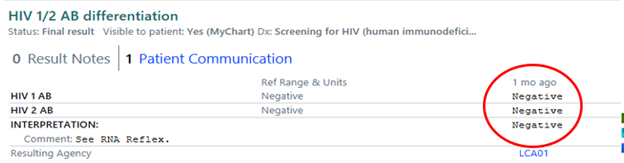
Above are the HIV 1/HIV 2 specific confirmatory tests done with the Geenius supplemental assay. It works more like the old Western blot and looks for several antibodies for each individual HIV type. This is a much more specific test. Prior to easy access of HIV viral loads, this is the way we always confirmed the HIV diagnosis. And, as such, it is a reliable method to rule in or rule out HIV. This test is always below the screening results and if it is negative as noted above, you can rest assured that the patient is negative for HIV and you can reassure the patient as well. No further testing is required.
The Infectious Diseases section is happy to answer your questions about HIV, treatment, testing and prevention but this may help you provide quick reassurance to your patients from test results that can generate a lot of anxiety.

